Introduction and Outline
When you ask a Materials Scientist of Engineer what types of materials they work on, many of them will give the response "soft materials". This may sounds a bit mysterious at first, but this is a core feature of any Materials Scientist's identity: whether they work with hard, stiff, strong stuff like metals and ceramics, or if they work with soft(er), more compliant, and weaker stuff like polymers.
The distinction arises immediately when we think about structure of these two broad classifications of materials. For "hard materials", we're typically looking at individual atoms packing into unit cells to form crystals - or alternatively atoms with ordered short-range interactions that have amorphous structure in the longer range: glasses. The behavior of these materials are determined to some large degree by the primary bonding between the atoms in the assemblies: ionic, covalent, or metallic.
In soft matter the situation is very different. Instead of atoms packing together in unit cells or creating covalent/ionic networks like in glasses, we have long chains of molecules interacting with each other via secondary interactions. These secondary interactions in "soft materials" are usually weaker than the interactions in their "hard material" counterparts, leading to lower melting temperatures, low thermal and electrical conductivity, and modest mechanical properties.
But, while the chemistry which dictates the interaction between the chains is important, the behavior of polymers extends well beyond the simple secondary interactions between these polymer chains. The chains themselves have structural (and chemical) characteristics, including their size, conformation (or shape), and architecture. This is similar to what we saw when studying metals and ceramics: it isn't just the bonds between the atoms that are important, but also other structural features such as degree of crystallinity and defect structure. These features are mapped visually in Figure 9.2.1.
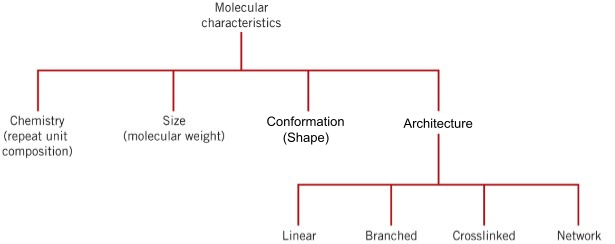
Figure 9.2.1 A map of the some of the main molecular characteristics that determine the behavior of polymers.
Outline
In this chapter, we'll explore the structural details shown in Figure 9.2.1 and show how these features can lead to changes in polymerica behavior.
- Section 9.2, Polymer Chemistry: We begin with the basics of polymers - their chemical makeup - and demonstrate how the polymer chain is a repeat unit of many mers with different chemical identities.
- Section 9.4, Polymer Conformation: Once we understand the chain-like structure that emerges due to the bonding of mers, we explore the conformation - or shape - of this structure using a random-walk
NetLogomodel. - Section 9.5, Polymer Synthesis: Once we understand the structures we can have in a chain, we take a step back and investigate how these chains may grow, focusing on the two most prominent types of polymerization reactions: chain-growth and step-growth.
- Section 9.6, Polymer Size: We'll see that during polymer synthesis we can produce chains of different sizes. We'll explore and quantify them here.
- Section 9.7, Polymer Architecture: Finally, we explore how polymer structures at a higher hierarchical level can form: polymer architectures. These include linear, branched, cross-linked, and networked.
Outcomes
- Recognize the features within polymer structure hierarchy (chemistry, size, shape, architecture) and compare simple properties (melting temperature, strength, density) of polymers with varying structures.
- Compute important values for polymer structures such as molecular weight, end-to-end distance, dispersity, etc.
- Utilize polymerization models to make basic predictions about molecular weight distributions.
- Explain the roles of primary and secondary bonding in polymeric structures.
- Discuss how polymers occupy space due to their intermolecular bonding, size, and conformation.
- Understand how simple random walk models lead to polymer conformation and conjecture about limitations and extensions of the model.
- Differentiate between various types of polymer synthesis (chain-growth/step growth) and evaluate how simple models of synthesis may influence polymer size and architecture.
- Correlate polymer architecture with post-consumer processability (recycling).